2018
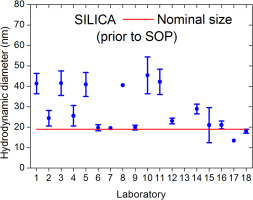
Nanoparticle in vitro toxicity studies often report contradictory results with one main reason being insufficient material characterization. In particular the characterization in biological media remains challenging. Our aim was to provide robust protocols for two of the most commonly applied techniques for particle sizing, i.e. dynamic light scattering (DLS) and differential centrifugal sedimentation (DCS) that should be readily applicable also for users not specialized in nanoparticle physico-chemical characterization. A large number of participants (40) were recruited for a series of inter-laboratory comparison (ILC) studies covering many different instrument types, commercial and custom-built, as another possible source of variation. ILCs were organized in a consecutive manner starting with dispersions in water employing well-characterized near-spherical silica nanoparticles (nominal 19 nm and 100 nm diameter) and two types of functionalized spherical polystyrene nanoparticles (nominal 50 nm diameter). At first each laboratory used their in-house established procedures. In particular for the 19 nm silica particles, the reproducibility of the methods was unacceptably high (reported results were between 10 nm and 50 nm). When comparing the results of the first ILC round it was observed that the DCS methods performed significantly worse than the DLS methods, thus emphasizing the need for standard operating procedures (SOPs). SOPs have been developed by four expert laboratories but were tested for robustness by a large number of users in a second ILC (11 for DLS and 4 for DCS). In a similar approach another SOP for complex biological fluids, i.e. cell culture medium containing serum was developed, again confirmed via an ILC with 8 DLS and participating laboratories.
Our study confirms that well-established and fit-for-purpose SOPs are indispensable for obtaining reliable and comparable particle size data. Our results also show that these SOPs must be optimized with respect to the intended measurement system (e.g. particle size technique, type of dispersant) and that they must be sufficiently detailed (e.g. avoiding ambiguity regarding measureand definition, etc.). SOPs may be developed by a small number of expert laboratories but for their widespread applicability they need to be verified by a larger number of laboratories.
2017
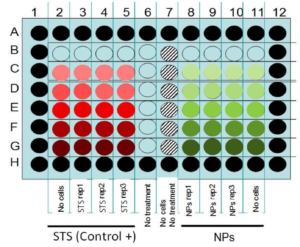
The rapid development of nanotechnologies and increased production and use of nanomaterials raise concerns about their potential toxic effects for human health and environment. To evaluate the biological effects of nanomaterials, a set of reliable and reproducible methods and development of standard operating procedures (SOPs) is required. In the framework of the European FP7 NanoValid project, three different cell viability assays (MTS, ATP content, and caspase-3/7 activity) with different readouts (absorbance, luminescence and fluorescence) and two immune assays (ELISA of pro-inflammatory cytokines IL1-β and TNF-α) were evaluated by inter-laboratory comparison. The aim was to determine the suitability and reliability of these assays for nanosafety assessment. Studies on silver and copper oxide nanoparticles (NPs) were performed, and SOPs for particle handling, cell culture, and in vitro assays were established or adapted. These SOPs give precise descriptions of assay procedures, cell culture/seeding conditions, NPs/positive control preparation and dilutions, experimental well plate preparation, and evaluation of NPs interference. The following conclusions can be highlighted from the pan-European inter-laboratory studies: Testing of NPs interference with the toxicity assays should always be conducted. Interference tests should be designed as close as possible to the cell exposure conditions. ATP and MTS assays gave consistent toxicity results with low inter-laboratory variability using Ag and CuO NPs and different cell lines and therefore, could be recommended for further validation and standardization. High inter-laboratory variability was observed for Caspase 3/7 assay and ELISA for IL1-β and TNF-α measurements.
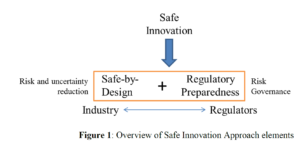
The Safe-by-Design (SbD) concept is already in use in different industrial sectors as an integral part of the innovation process management. However, the adopted approach is often limited to design solutions aiming at hazard reduction. Safety is not always considered during the innovation process, mainly due to the lack of knowledge (e.g. in small and medium companies, SMEs) and the lack of dialogue between actors along the innovation chain. The net result is that safety is considered only at the end of the innovation process at the market authorization phase, with potential loss of time and money. This is especially valid for manufactured nanomaterials (MNM) for which the regulatory context is not completely developed, and the safety knowledge is not readily available. In order to contribute to a sustainable innovation process in the nanotechnology field by maximising both benefits and safety, the NANoREG project developed a Safe Innovation approach, based on two elements: the Safe-by-Design approach which aims at including risk assessment into all innovation stages; and the Regulatory Preparedness, focused on the dialogue with stakeholders along the innovation chain. In this work we present some examples about the implementation in our Laboratory of this approach for different MNM applications, covering different steps of the innovation chain. The case studies include: the feasibility study of a medical device including substances, for topical application; the testing of two potential nanotech solutions for the consolidation of cultural heritage artifacts; the testing of coatings already on the market for other uses, which was tested as food contact materials (FCM) to evaluate the conformity to food applications. These three examples represent a good opportunity to show the importance of NANoREG SbD and Safe Innovation Approach in general, for developing new nanotechnology-based products, also highlighting the crucial role of EU ProSafe project in promoting this concept to industries and interested stakeholders.
doi :10.1088/1742-6596/838/1/012019
In this paper we present an electrochemical approach to prepare standard solutions of metal ions in a room temperature ionic liquid (IL), which can find useful application for analysis in hydrophobic matrices. The method, developed here for the case of lead ions, is based on the galvanostatic dissolution of a lead anode dipped directly in a suitable IL, namely tri-hexyl(tetradecyl)phosphonium bis (trifluoromethylsulfonyl) imide ([P14,6,6,6]+[NTf2]–). After each oxidation step, the metal dissolution process in the IL solutions was monitored by cyclic voltammetric measurements at a glassy carbon disk electrode. The results indicated that the peak current relevant to the reduction of the electro-generated Pb(II) increased linearly while increasing the oxidation time. By varying the oxidation time from 200 to 6000s, a set of Pb(II)/[P14,6,6,6]+[NTf2]– solutions at concentrations ranging between 10 and 300μgg-1 was prepared. To validate the efficiency of the electrochemical procedure to produce metal ion standard solutions, the Pb content was quantified by developing a microwave digestion procedure specifically suitable for the IL medium, followed by ICP-QMS analysis in the digested standards. The results indicated a satisfactory agreement between concentrations found by ICP-QMS and calculated from electrochemical data, with a coulometric efficiency of Pb(II) generation in ionic liquid ≥95.6%. Finally, the applicability of the Pb(II)/IL solutions as standards for analyses in hydrophobic media was tested by determining, by ICP-QMS, the Pb content in an extra-virgin olive oil spiked with known amounts of a Pb(II)/IL standard. Satisfactory Pb recoveries, ≥96%, were measured.
https://doi.org/10.1016/j.talanta.2017.05.039
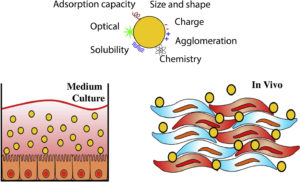
The perceived enormous potential of nanotechnology in contributing to sustainable innovation has led to the growth of investments into new industrial applications and consumer products. However, the lack of tools that are needed to generate early knowledge about the potential adverse effects, combined with the uncertainties regarding the health and safety risks of engineered nanoparticles (ENPs), are a potential threat to the acceptability by society of the nanotechnology innovations, due to the rising societal concerns that are based on generic worries. In order to tackle these issues, it has been necessary to adopt a more proactive approach into nanotechnology safety assessments. Multiple projects have been initiated around the world in order to understand how ENPs interact with living organisms, but the validation of most of the emerging knowledge may take years. This is while robust risk assessment results are urgently needed, in order to support timely regulatory decisions and risk management actions. The goal of this paper has been to review the present knowledge on the physicochemical characteristics of ENPs, focusing on titanium dioxide(TiO2), gold (Au), copper oxide (CuO), and zinc oxide (ZnO), as well as on their biological interactions. In addition, the paper has been aimed at the identification of the main challenges on the current toxicological characterisation of these ENPs. Focus will also be given in this article to those ENPs that have been described by the Consumer Product Inventory as having prevalent nanomaterials present in consumer products, but also, with those having therapeutic and diagnostic applications, due to their physical (ex: confined plasmon resonances) and biological (biocompatibility and antimicrobial) properties.
Nanomedicine opens new frontiers for treating, curing and diagnosing diseases due to the unique physico-chemical properties of nanomaterials. Nano-based drug delivery systems are one of main applications of nanomedicine. To develop promising nano-based drug delivery systems, both efficacy and safety should be carefully evaluated. However, at present, a rational and solid approach in response to these needs has not been devised. In the present work we present a novel testing strategy, based on in vitro cell models and approaches, aimed to evaluate the safety of orally administered nanocarriers, emphasizing the importance of harmonized and validated standard operating procedures in a safe-by-design context.
link: approach-for-in-vitro-testing-of-oral-nanocarrier-toxicity
The many interesting physical and chemical properties of carbon nanotubes (CNT) make it one of the most commercially attractive materials in the era of nanotechnology. Here, we review the recent publications on in vivo biodistribution of pristine and functionalized forms of single-walled and multi-walled CNT. Pristine CNT remain in the lung for months or even years after pulmonary deposition. If cleared, the majority of CNT move to the gastrointestinal (GI) tract via the mucociliary escalator. However, there appears to be no uptake of CNT from the GI tract, with a possible exception of the smallest functionalized SWCNT. Importantly, a significant fraction of CNT translocate from the alveolar space to the near pulmonary region including lymph nodes, subpleura and pleura (<7% of the pulmonary deposited dose) and to distal organs including liver, spleen and bone marrow (~1%). These results clearly demonstrate the main sites of long-term CNT accumulation, which also includes pleura, a major site for fibre-induced pulmonary diseases. Studies on intravenous injection show that CNT in blood circulation are cleared relatively fast with a half-life of minutes or hours. The major target organs were the same as identified after pulmonary exposure with the exception of urine excretion of especially functionalized SWCNT and accumulation in lung tissue. Overall, there is evidence that CNT will primarily be distributed to the liver where they appear to be present at least one year after exposure.
https://doi.org/10.1111/bcpt.12705
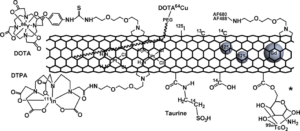
Examples of the various indirect detections of SWCNT/MWCNT. The illustration is based on drawings and/or descriptions in the below-mentioned biodistribution papers. AF488 and AF680 are Alexa flour fluorophores. DOTA, 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid; DTPA, diethylen-triamine-penta-acetic dianhydride. There are different examples of how DOTA and DTPA are attached to the CNT and of which tracers are used.
2016
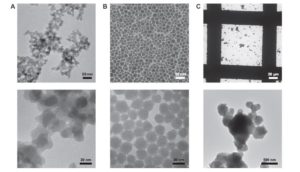
Silver nanoparticles (AgNPs) are increasingly used in medical devices as innovative antibacterial agents, but no data are currently available on their chemical transformations and fate in vivo in the human body, particularly on their potential to reach the circulatory system. To study the processes involving AgNPs in human plasma and blood, we developed an analytical method based on hydrodynamic chromatography (HDC) coupled to inductively coupled plasma mass spectrometry (ICP-MS) in single-particle detection mode. An innovative algorithm was implemented to deconvolute the signals of dissolved Ag and AgNPs and to extrapolate a multiparametric characterization of the particles in the same chromatogram. From a single injection, the method provides the concentration of dissolved Ag and the distribution of AgNPs in terms of hydrodynamic diameter, mass-derived diameter, number and mass concentration. This analytical approach is robust and suitable to study quantitatively the dynamics and kinetics of AgNPs in complex biological fluids, including processes such as agglomeration, dissolution and formation of protein coronas. The method was applied to study the transformations of AgNP standards and an AgNP-coated dressing in human plasma, supported by micro X-ray fluorescence (μXRF) and micro X-ray absorption near-edge spectroscopy (μXANES) speciation analysis and imaging, and to investigate, for the first time, the possible presence of AgNPs in the blood of three burn patients treated with the same dressing. Together with our previous studies, the results strongly support the hypothesis that the systemic mobilization of the metal after topical administration of AgNPs is driven by their dissolution in situ
The fascinating properties of nanomaterials opened new frontiers in medicine. Nanocarriers are useful systems in transporting drugs to site-specific targets. The unique physico-chemical characteristics making nanocarriers promising devices to treat diseases may also be responsible for potential adverse effects. In order to develop functional nano-based drug delivery systems, efficacy and safety should be carefully evaluated. To date, no common testing strategy to address nanomaterial toxicological challenges has been generated. Different cell culture models are currently used to evaluate nanocarrier safety using conventional in vitro assays, but overall they have generated a huge amount of conflicting data. In this review we describe state-of-the-art approaches for in vitro testing of orally administered nanocarriers, highlighting the importance of developing harmonized and validated standard operating procedures. These procedures should be applied in a safe-by-design context with the aim to reduce and/or eliminate the uncertainties and risks associated with nanomedicine development.
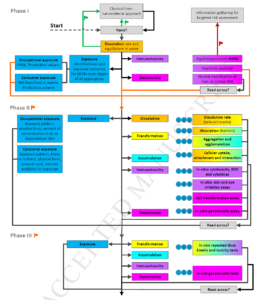
In the current paper, a new strategy for risk assessment of nanomaterials is described, which builds upon previous project outcomes and is developed within the FP7 NANoREG project. NANoREG has the aim to develop, for the long term, new testing strategies adapted to a high number of nanomaterials where many factors can affect their environmental and health impact. In the proposed risk assessment strategy, approaches for (Quantitative) Structure Activity Relationships ((Q)SARs), grouping and read-across are integrated and expanded to guide the user how to prioritise those nanomaterial applications that may lead to high risks for human health. Furthermore, those aspects of exposure, kinetics and hazard assessment that are most likely to be influenced by the nanospecific properties of the material under assessment are identified. These aspects are summarised in six elements, which play a key role in the strategy: exposure potential, dissolution, nanomaterial transformation, accumulation, genotoxicity and immunotoxicity.
With the current approach it is possible to identify those situations where the use of nanospecific grouping, read-across and (Q)SAR tools is likely to become feasible in the future, and to point towards the generation of the type of data that is needed for scientific justification, which may lead to regulatory acceptance of nanospecific applications of these tools.
The global nutraceutical product market is continuously growing, and nanotechnology represents an innovative way to effectively increase oral bioaccessibility and bioavailability of active ingredients, preserving them from the digestive process. In spite of benefits, there is an urgent need for regulatory systems capable of managing risks associated with nanofoods and the use of nanotechnology in the food industry. In this chapter, current international and European regulatory aspects are addressed. Special attention is given to the European Union regulatory framework, with the upcoming new definition of nanomaterials and the subsequent modification of existing regulations and declaration of new laws. To comply with existing directives and regulations for marketing of new nanotechnology-based products, European Food Safety Authority guidelines for assessing potential risks from nanotechnologies in the food sector are described.
https://doi.org/10.1016/B978-0-12-804305-9.00006-3
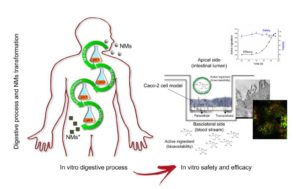
Schematic representation of the in vitro digestive process and Caco-2 system for evaluating safety and efficacy of nanotechnologybased
nutraceuticals.
Migration of nanomaterials from food containers into food is a matter of concern because of the potential risk for exposed consumers. The aims of this study were to evaluate silver migration from a commercially available food packaging containing silver nanoparticles into a real food matrix (chicken meat) under plausible domestic storage conditions and to test the contribution of such packaging to limit food spoilage bacteria proliferation. Chemical analysis revealed the absence of silver in chicken meatballs under the experimental conditions in compliance with current European Union legislation, which establishes a maximum level of 0.010 mg kg(-1) for the migration of non-authorised substances through a functional barrier (Commission Regulation (EU) No. 10/2011). On the other hand, microbiological tests (total microbial count, Pseudomonas spp. and Enterobacteriaceae) showed no relevant difference in the tested bacteria levels between meatballs stored in silver-nanoparticle plastic bags or control bags. This study shows the importance of testing food packaging not only to verify potential silver migration as an indicator of potential nanoparticle migration, but also to evaluate the benefits in terms of food preservation so as to avoid unjustified usage of silver nanoparticles and possible negative impacts on the environment.
doi: 10.1080/19440049.2016.1179794
Introduction: Increased use of nanomaterials has raised concerns about the potential for undesirable human health and environmental effects. Releases into the air may occur and, therefore, the inhalation route is of specific interest. Here we tested copper oxide nanoparticles (CuO NPs) after repeated inhalation as hazard data for this material and exposure route is currently lacking for risk assessment. Methods: Rats were exposed nose-only to a single exposure concentration and by varying the exposure time, different dose levels were obtained (C × T protocol). The dose is expressed as 6 h-concentration equivalents of 0, 0.6, 2.4, 3.3, 6.3, and 13.2 mg/m3 CuO NPs, with a primary particle size of 10 9.2–14 nm and an MMAD of 1.5 μm. Results: Twenty-four hours after a 5-d exposure, dose-dependent lung inflammation and cytotoxicity were observed. Histopathological examinations indicated alveolitis, bronchiolitis, vacuolation of the respiratory epithelium, and emphysema in the lung starting at 2.4 mg/m3. After a recovery period of 22 d, limited inflammation was still observed, but only at the highest dose of 13.2 mg/m3. The olfactory epithelium in the nose degenerated 24 h after exposure to 6.3 and 13.2 mg/m3, but this was restored after 22 d. No histopathological changes were detected in the brain, olfactory bulb, spleen, kidney and liver. Conclusion: A 5-d, 6-h/day exposure equivalent to an aerosol of agglomerated CuO NPs resulted in a dose-dependent toxicity in rats, which almost completely resolved during a 3-week post-exposure period.
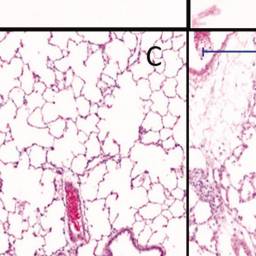
Histopathology of (A) control animal at day 6 with normal olfactory epithelium (10× magnification), (B) degeneration of the nose olfactory epithelium at day 6 at a 6-h concentration equivalent of 13.2 mg/m3 (10× magnification), (C) control animal at day 6 with normal lung alveoli (5× magnification), (D) lung alveolitis at day 6 after exposure to 13.2 mg/m3 (5× magnification), (E) minimal residual inflammatory reaction after recovery period of three weeks 22 d post-exposure to 13.2 mg/m3. Note: cellularity in alveoli and thickened alveolar walls (20× magnification). (F) Minimal residual inflammatory reaction after recovery period of three weeks 22 d post-exposure to 13.2 mg/m3. Note: alveolar macrophages in alveoli at 20× magnification.
Total (Ast), inorganic arsenic (Asi = As(III) + As(V)) and dimethylarsonic acid (DMA) were determined in 37 commercial rice samples collected in France. Ast was measured by inductively coupled plasma-mass spectrometry (ICP-MS) whereas anion-exchange chromatography – ICP-MS was used for Asi and DMA determination. Ast in raw rice varied from 0.041 to 0.535 mg kg−1 whereas Asi varied from 0.025 mg kg−1 (polished Basmati rice) up to 0.471 mg kg−1 (organic rice duo). The daily intake and associated health risk for different population groups as a function of age and gender was also assessed. The intake varied between 0.002 and 0.184 μg kg−1 body weight for Ast and 0.002 and 0.153 μg kg−1body weight for Asi, which do not pose a chronic toxicity risk. Organic wholegrain rice may entail a risk for children in the case of sole consumption at the expense of polished rice. The impact of rice cooking/boiling in terms of the overall toxicological risk related to As species was also investigated. Pre-rinsing and boiling the raw rice by using an excess of water is the most efficient mode to obtain a significant Asi removal and further reduction of the toxicological risk for children, particularly for white rice varieties.
2015
The use of nanotechnology in nutraceuticals is rapidly growing due to their ability to improve active ingredient solubility, stability and bioavailability. Their unique properties are mainly conferred by small size and high surface-to-volume ratio. However, these physico-chemical properties are also responsible for potential adverse effects of nanomaterials on human health. In order to develop new marketable nutraceutical formulations, the evaluation of their efficacy and safety is required. In this work, we present an in vitro-based approach to simultaneously evaluate efficacy and safety of new formulations in agreement with European Food Safety Authority recommendations and in high-throughput screening fashion. This approach is time and costs effectiveness, providing a useful support to Companies since it well fits with the safe-by-design concept that is currently under development in Europe.
http://www.teknoscienze.com/tks_article/nutraceuticalsand-nanotechnology/
Works of art are constantly under physical, chemical and biological degradation, so constant restoration is required. Consolidation is an important step in restoration, and traditional approaches and materials have already shown their limitations. To solve these problems, new nanoparticle-based consolidants were developed. No information on their toxicity is yet available. In this work, we focused our attention on potential risks posed by three commercially available nanoparticle-based consolidants: silica (SiO2 NPs), silanized silica (silanized SiO2 NPs) and calcium hydroxide (nanolime) nanoparticle dispersions. Occupational exposure impact was tested on three in vitro models mimicking inhalation, dermal contact and systemic routes. While no toxic effects were observed for nanolime and silanized SiO2 NPs, bare SiO2 NPs showed a dose- and time-dependent damage in all considered models. Corrosion test on EpiSkin® revealed no viability reduction. Works of art degradation is partially due to microorganism activity. Consolidant antibacterial activity was evaluated on three representative bacterial strains. Silica NPs-based consolidants showed effect on specific bacterial groups, while no specificity was observed with nanolime. In conclusion, silanized SiO2 NPs-based consolidant emerged as the safest and bacteriologically active product. The different biological impact of bare and silanized SiO2 NPs highlights the importance of safe-by-design approach in developing nanoparticle-containing products.
2014
The growing use of engineered nanomaterials in both commercial products and biomedical applications leads to increasing exposure for production line workers, consumers and patients. Therefore, the understanding of biological effects induced by nanomaterials is crucial for their safety assessment. An important group of nanomaterials is represented by metal(loid)-based nanoparticles, because of their unique physico-chemical properties. Metal(loid)-based nanoparticles themselves, the related ion release, and their nanometallomes, can potentially interact with essential elements causing dys-homeostasis and adverse biological effects. In this work, we describe the effects of metal(loid)-based nanoparticles on essential element homeostasis. In particular, we consider the most used and promising metal(loid)-based nanoparticles, highlighting that the new emerging concept of nanometallomics is important to disclose the impact of these nanoparticles on human health and the related long-term effects.
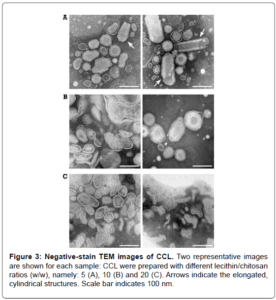
Among nanoparticle-based drug delivery formulations, lecithin/chitosan liposomes are promising candidates because of their biocompatibility, biodegradability and bioadhesion properties. Lecithin is a mixture of highly biocompatible phospholipids, while chitosan represents one of the most used polymers in pharmaceutical formulations. Their combination results in positively charged complexes that are able to sustain a specific, prolonged and controlled release. Scarce reproducibility and batch-to-batch variation in lecithin/chitosan liposome synthesis, as well as difficult scale-up to industrial production, is a major challenge for their utilization. Here we present a strictly controlled procedure, based on ethanol diffusion in water, which yields to a precise and reproducible self-organization of lecithin and chitosan molecules. We analysed the size, surface charge and stability of chitosan coated liposomes at different lecithin/chitosan ratios. We found that increasing the lecithin/chitosan ratio both mean particle size and surface charge were progressively reduced. A good stability was observed for all formulations, though interactions occurred in the presence of low amount of surface-adsorbed chitosan liposome vesicle. Chitosan coated liposomes interact with A549 and Caco-2 cells inducing low toxic effects only with prolonged incubation times. In conclusion, the proposed procedure provides good reproducibility in the formation of non-toxic and highly stable formulations of chitosan coated liposomes as drug delivery systems.
2013
The increasing production volumes and commercialization of engineered nanomaterials (ENM), together with data on their higher biological reactivity when compared to bulk counterpart and ability to cross biological barriers, have caused concerns about their potential impacts on the health and safety of both humans and the environment. A multidisciplinary component of the scientific community has been called to evaluate the real risks associated with the use of products containing ENM, and is today in the process of developing specific definitions and testing strategies for nanomaterials. At ECSIN we are developing an integrated multidisciplinary methodological approach for the evaluation of the biological effects of ENM on the environment and human health. While our testing strategy agrees with the most widely advanced line of work at the European level, the choice of methods and optimization of protocols is made with an extended treatment of details. Our attention to the methodological and technical details is based on the acknowledgment that the innovative characteristics of matter at the nano-size range may influence the existing testing methods in a partially unpredictable manner, an aspect which is frequently recognized at the discussion level but oftentimes disregarded at the laboratory bench level. This work outlines the most important steps of our testing approach. In particular, each step will be briefly discussed in terms of potential technical and methodological pitfalls that we have encountered, and which are often ignored in nanotoxicology research. The final aim is to draw attention to the need of preliminary studies in developing reliable tests, a crucial aspect to confirm the suitability of the chosen analytical and toxicological methods to be used for the specific tested nanoparticle, and to express the idea that in nanotoxicology,”devil is in the detail”.